Posts Tagged ‘nanotechnology’
Nanopollution in the Air
In an article a few weeks ago, I noted the way the synthetic particle cerium oxide had a tendency to stick around in incinerators, seeming indestructible. While worrying, this was not an immediate concern because cerium oxide is non-toxic, though it did suggest the need for more rigorous testing of all the chemicals we are putting into products – especially nano-scale synthetics that might pose a particular clean-up problem.
Well, research from Trinity College in Dublin might show that we’re already behind the curve on this. You can read the phys.org article on it here. Basically, the research showed that exposure carbon-based and silicon-based nanotechnology, including carbon nanotubes, can lead to rheumatoid arthritis and autoimmune diseases, as established by tests on human respiratory cells and on mice.
So, we now know that much of the new nanotechnology being developed may have serious health impacts. What we don’t know is where all the particles are going to end up or how easy or difficult it might be to clean it all up. Since many of the chemicals being developed are proprietary and treated as corporate secrets, there’s no guarantee that we’ll know what the new materials might be used in.
As the Guardian notes here, nanotechnology exists in a gaping hole in the regulatory framework, and companies are taking full advantage of the fact. Analysis of potential risks is virtually nonexistent. The question is, can university research money going to studies like that from Trinity College in Dublin keep up with the research and development money in the private sector. I think we know what the answer is.
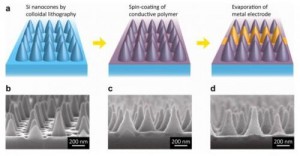
Nanocones for Cheap Solar Cells
This dovetails on the article about the solar-powered airplane. While the efficiency of solar panels is critical to making them worth deploying as an alternative to fossil fuels, that’s only half the issue. The other half, of course, is price. Right now, the cost is a dollar a watt, which is pretty hefty. Fortunately, increasing efficiency and decreasing price go hand-in-hand. In an earlier article, we introduced the idea that adding wrinkles might make the cells more efficient, and therefore cheaper. This time, it’s a different method of increasing surface area: cones.
Of course, because these are really small cones, they are dubbed “nanocones,” and the Stanford University study was published in “Nano Letters.” There will come a point where we will have to stop using nano- to prefix to everything that’s in the nanometer range, since most technology already incorporates components in that range, and in the near future, all of it will. We’ll need to be a bit more specific. After all, we already have carbon nanocones, which are altogether different in application from these silicon cones. But that’s just a pet-peeve of mine – for now, nano’s captured everyone’s imagination, so I guess we’ll go with it everywhere we can.
The key to the cost-savings of this design is the space between the cones. In a normal polymer cell, you need a full second layer for the polymer, but with this design, it can simply fill the gaps. Having a second layer involves using other materials, so this method renders those unnecessary.
On the Persistence of Nano-waste
First of all, a clarification. The prefix nano- seems to get attached to all sorts of stuff these days, especially in the science press, and it’s just begging for confusion. Nano-waste simply refers to synthetic particles on the nanometer scale that are engineered for some purpose, and are discarded along with the product when it has outlived its usefulness. It is nanotechnology in the broadest sense of the term – and we really need to be clearer about what we mean by nanotechnology going forward – though the conclusions reached by Walser, T. et. al. in the journal Nature Nanotechnology certainly has relevance to applications of small-scale computing (what we normally think about when we discuss nanotech) as well.
The particular problem raised by Walser, et al is that cerium oxide, a non-toxic synthetic used in catalytic converters, neither burns up nor biodegrades, so that it just sticks around and may eventually end up in our water supply and food cycle. Try to send it through an incinerator, and it will end up with the residue and tossed into a landfill, where it will persist indefinitely.
The good news is that cerium oxide is non-toxic and doesn’t escape into the atmosphere through the incineration process. The bad news is that it is not the only synthetic we have or are developing, and in many cases the environmental impact of the new compounds is not adequately tested. Once researchers find a practical use for them, they’re put to work without regard for whether we can get rid of them, or what they might do to us once they end their useful life and wind up in a pile of waste.
DNA Computing – Helix Memory Storage
I don’t think many people have any idea how far we’ve come. Because I like to keep track of scientific and technological developments as part of writing science fiction, I have a better idea than most, but then a story like this one comes across my desktop and I’m left in disbelief.
It has only been sixty years since the structure of DNA was understood, and now researchers at Stanford University have figured out how to write, read, and rewrite bits of information on DNA itself, making DNA an information storage device. When I say bits, I mean bits – they haven’t managed a full byte (eight bits) yet.
Still, it’s pretty amazing. They use two proteins – integrase and excisionase – and by getting the balance between the two right, can reliably switch the direction of a section of DNA. The DNA in question was within the chromosomes of the favorite of biological researchers – E. coli.
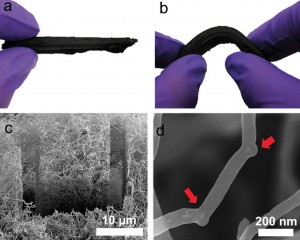
Cleaning Oil Spills with Nanotube Sponges
Of all materials of the future, few have captured the imagination more than carbon nanotubes. Science fiction fans have seen nanotubes as the key to building the stuff of our dreams – from space elevators to cyborg-like synthetic muscles. At the same time, practical, everyday uses have been popping up all over the place, creating a list so long in needed its own Wikipedia page.
Well, add another one to the list: cleaning up oil spills. It turns out that, if you add boron into the mix, it creates elbows in the tubes. So, instead of the traditional one-dimensional alignment we’ve already been getting so much fun out of, the fibers get connected together into a three dimensional sponge. Having created a sponge, scientists logically put it to work doing what sponge-like structures do best – soaking stuff up. Made up of carbon, it attracts oil and repels water, and it can absorb up to 100 times its weight in oil according to Bobby Sumpter at the Oak Ridge National Laboratory.